Engineered for delicate skin.
Validated for parent trust.
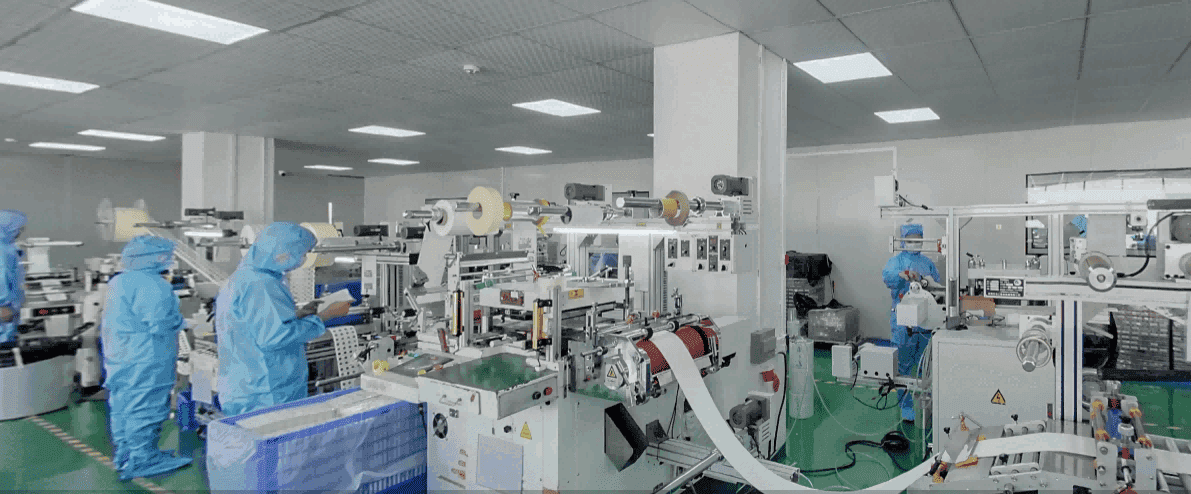
Manufacturing pediatric nasal strips and gentle mouth tapes with uncompromising adherence to safety standards. ISO 13485 compliant.

Audited & Certified Manufacturing Standards





Pediatric Indications & Use Cases
Gentle, safe, and effective solutions designed specifically for younger patients.

Nasal Congestion
Mechanical dilation for improved airflow during sleep, helping children breathe easier without medication.
Mouth Breathing
Gentle habit correction with safety-first central vents to ensure oral safety while promoting nasal breathing.

Sensitive Dermis
Specially formulated for developing skin barriers using hypoallergenic, latex-free medical grade materials.

Supervised Use
Designed for safe application and removal by parents or guardians to ensure proper placement and comfort.
Laboratory Verified Safety
We don't just claim "gentle" — we prove it. Every batch is subject to rigorous biological evaluation standards (ISO 10993) to ensure pediatric biocompatibility.
Cytotoxicity Tested
Passed ISO 13485 standards. Materials show zero toxic effect on cell cultures, ensuring cellular safety for long-term skin contact.
Skin Sensitization
Verified under ISO 13485. Formulated to minimize potential for allergic reaction or delayed hypersensitivity in children.

Irritation Free
Primary Skin Irritation tests confirm < 0.4 index score. Safe for repeated nightly application on delicate facial areas.
Full Traceability
Every production lot includes COA (Certificate of Analysis) and raw material tracing to ensure no cross-contamination.
Biocompatible Material Selection
We utilize exclusively medical-grade substrates free from common allergens. No latex, no parabens, no fragrance.

Hypoallergenic Non-Woven
High porosity ensures skin respiration during overnight wear.

Pure Medical Cotton
Natural fiber option for maximum softness and minimal environmental impact.

Soft-Touch Synthetic
Smooth, friction-free surface designed to prevent snagging on bedding.
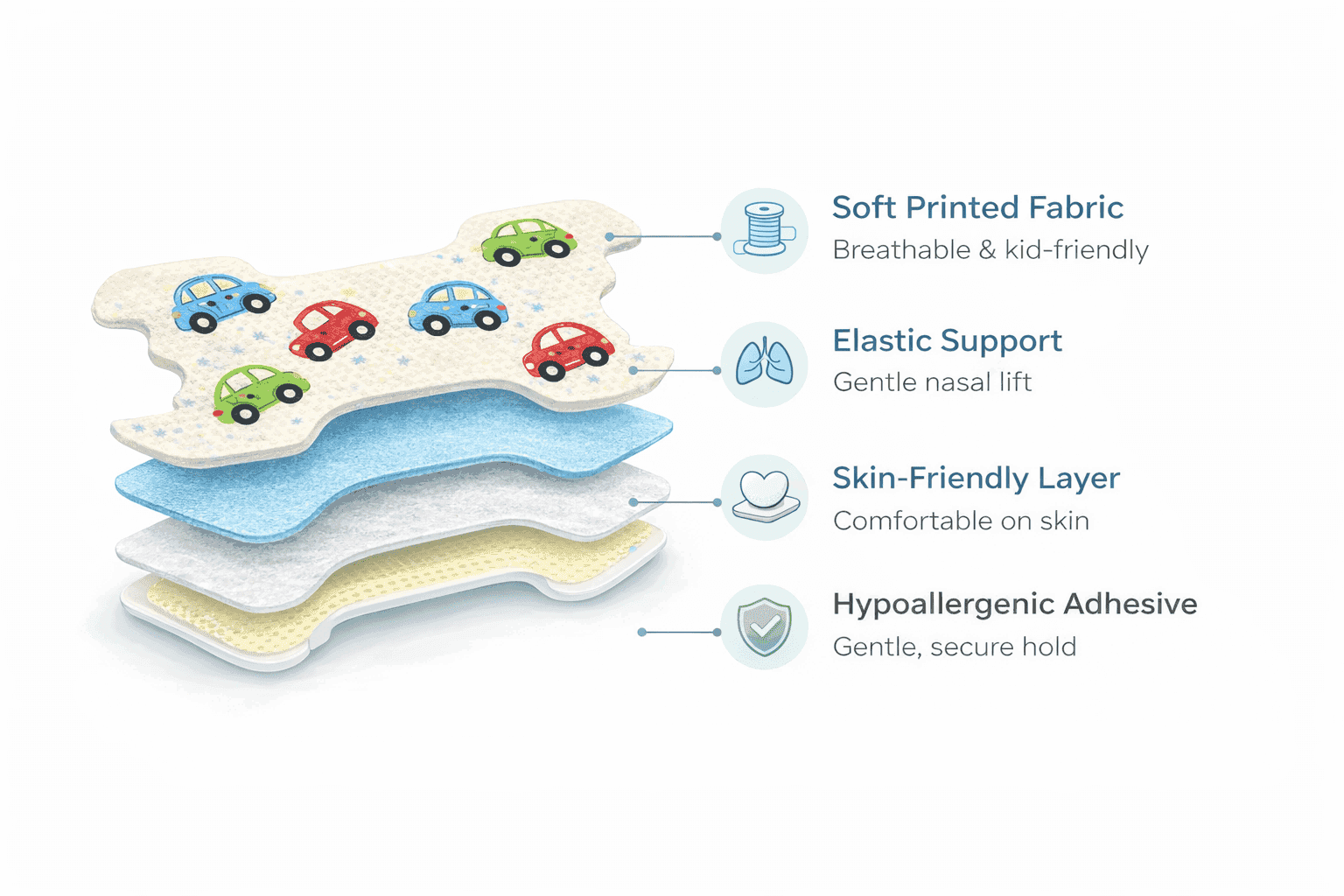
Adhesion Engineered for Pain-Free Removal
Adult adhesives can cause trauma to pediatric skin. Our specific pediatric formulation reduces peel force by 35% compared to standard strips, while maintaining sufficient hold for 8-10 hours.
Solvent-Free Gel Acrylic
Eliminates harsh chemical residue.
Micro-Porous Structure
Allows moisture evaporation to prevent skin maceration.
Risk Mitigation & Quality Assurance
We prioritize regulatory compliance to protect your brand from liability and platform policy violations.
Choke Hazard Prevention
Single-piece construction with no detachable small parts.
Biocompatibility
Passed ISO 13485 testing for cytotoxicity and sensitization.
Airflow Vents
Mouth tapes feature mandatory central breathing vents for safety.
Platform Compliance
Documentation ready for Amazon Kids & Medical categories.
Supply Chain Reliability
From pilot runs to mass retail fulfillment.
Automated 100% surface defect scanning.
Clean-room packing to prevent contamination.
End-to-end shipping options.
Pediatric Nasal Strip Design Styles
A selection of child-safe nasal strip designs engineered for comfort, stable fit, and low-irritation removal.

Butterfly Fit (Rounded Edge)
Ref: PED-DES-01
- Rounded corners reduce edge lifting.
- Balanced spring force.

Ergonomic Oval (Sensitive Care)
Ref: PED-DES-02
- Reduced edge contact.
- Lower-profile design.

Compact Support (Stable Hold)
Ref: PED-DES-03
- Compact shape minimizes movement.
- Secure without aggressive adhesion.
Pediatric Mouth Tape Design Styles
Conservative, safety-first designs engineered to avoid full-seal coverage.

X-Vent Safety Design
Ref: PED-MT-DES-01
- Avoids full-seal coverage.
- Low-tack adhesion.

O-Vent (Central Air Passage)
Ref: PED-MT-DES-02
- Central opening for airflow.
- Rounded perimeter.

Two-Tab Comfort Design
Ref: PED-MT-DES-03
- Reduced coverage.
- Easier alignment.
Retail-Ready Packaging
Parents buy with their eyes, but they trust with information. We design packaging that balances shelf-appeal with necessary medical warnings and usage instructions.
- ✓ Tamper-evident seals
- ✓ Clear age-grading display
- ✓ Multi-language instruction leaflets

Common Safety & Compliance Queries
Q.
How does the adhesive differ from adult nasal strips?
+
Q.
Are your mouth tapes compliant?
+
Q.
What safety features do you recommend for kids mouth tape (vents, frames, or cut-outs)?
+
Q.
Can you provide biocompatibility or skin-safety documentation for sensitive pediatric users?
+
Q.
How do you prevent skin trauma during removal (MARSI) for kids?
+
Q.
What is the MOQ for pediatric sizes and custom shapes?
+
Q.
Can you support retail packaging requirements (hang hole, multi-language, warnings) for kids products?
+
Q.
How do you ensure batch-to-batch consistency for pediatric programs?
+
Eliminate Sourcing Risks.
Scale with Confidence.

The Reality
Cheap suppliers mean Amazon takedowns and 1-star reviews.
We are different. We are a certified medical factory providing the documentation you need to win.
Full Compliance
FDA, CE & ISO 13485 ready.
Low MOQ Start
Pilot with just 500 units.
Hypoallergenic
Medical acrylic & silicone gel.
Rapid Speed
7-day prototyping turnaround.
Certified Manufacturing Standards
Request OEM Quote
Tell us about your project. We typically reply within 12 hours with a price list and sample options.